What’s New at AAPM?
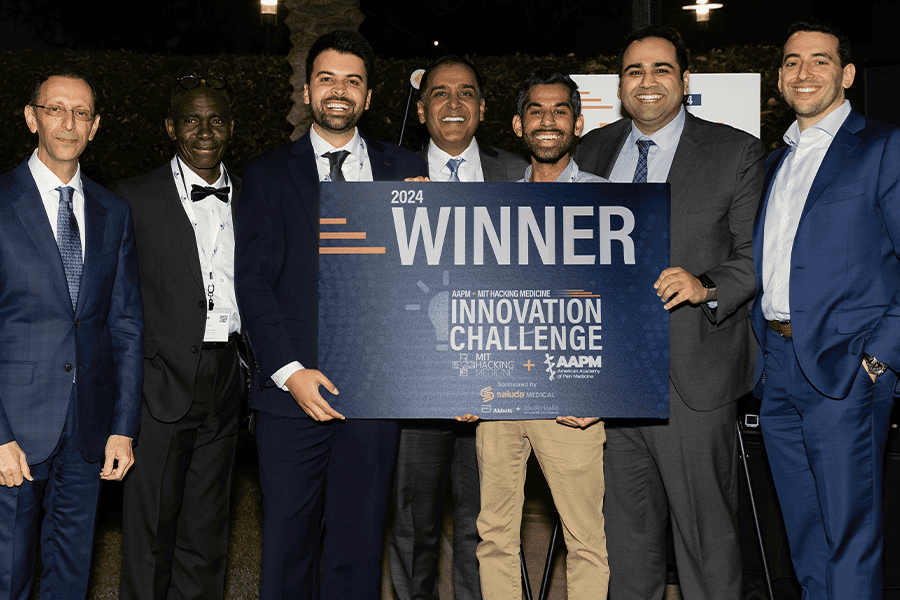
Innovators Propel Pain Medicine Forward at the 3rd Annual Innovation Challenge
Menda Health co-founders along with AAPM leadership and Innovation Challenge co-founders. This year's 3rd Annual Innovation Challenge Presented by AAPM + [...]
Celebrating 40 Years of Innovation and Collaboration at the AAPM 2024 Annual Meeting
More than 600 attendees converged in Scottsdale, AZ, for our milestone 40th Annual AAPM Meeting. Welcoming a range of multidisciplinary pain medicine specialists, [...]
Honoring Our 2024 Presidential Commendation Awardees
Join us in recognizing the 2024 Presidential Commendation Awardees. From groundbreaking education initiatives to vital policy advancements, these individuals and teams are shaping [...]
How Can AAPM Help You?
MEMBER BENEFITS
Why join AAPM?
Tap into an expansive network of professional pain medicine clinicians with a sustained interest in the multidisciplinary approach to pain disorders and their management.
CAREERS
Connecting top talent in pain medicine careers
Find or become a candidate for that next step up in your pain medicine career with our comprehensive database of job opportunities.
JOURNAL
Access to the industry’s leading scholarly journal on the science of pain medicine
Stay current with leading trends in the multidisciplinary approach to pain medicine and share your thought leadership with peers.
EVENTS
We’re better together, finding solutions through events that matter to our industry
Learn and experience more in the multidisciplinary field of pain medicine through conferences, webinars, and networking events.
Pain Matters Podcast
The Pain Matters Podcast is presented by the American Academy of Pain Medicine. It’s the nation’s leading podcast for healthcare providers focused on providing the best care today, tomorrow, and beyond. Each episode, we’ll share the latest innovations and practical applications that directly impact how we care for patients and measure success in multidisciplinary care.
Sign Up for the AAPM Newsletter
Get the latest news, events, and updates from AAPM delivered straight to your inbox.